On March 2, 2021, Daniel Seara successfully defended his thesis “Energetics of biological mechanics and dynamics” (Advisor: Michael Murrell).
Seara explained, “Over the past century, heroic work by molecular biologists has not only uncovered the key players in various biological processes, from protein synthesis to cell division, but also revealed the importance of consumption of energy for their proper functioning. However, we lack general rules connecting energy dissipation and biological function. In my thesis, I developed techniques for estimating energy dissipation from biological experiments by measuring how irreversible the observed dynamics are. Across various systems, we find that dissipation can characterize dynamical and mechanical phase transitions in living matter. This work provides a framework with which we can begin to unify seemingly disparate biological phenomena using non-equilibrium statistical physics.”
Seara will be a Postdoctoral Fellow at the University of Chicago.
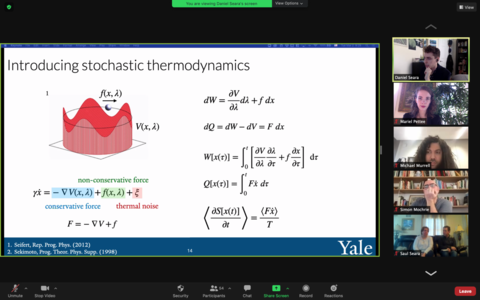
Thesis Abstract: Living matter is a class of soft matter systems that microscopically consumes energy to drive essential life processes such as replication, migration, and shape change at the scale of both single cells and multicellular tissues. While much work has been done to understand the molecular processes underlying such behaviors, we lack a general understanding of how the microscopic breaking of detailed balance translates to large-scale cellular behaviors and materials properties. Using the tools of stochastic thermodynamics, this thesis uses energy dissipation to understand dynamical and mechanical phase transitions driven by force generation, binding kinetics, and (dis)assembly seen in experiments, simulations, and theoretical models of biological materials. We focus on the actomyosin cytoskeleton, a network composed of polymeric proteins (actin) subject to forces molecular motors (myosin), as our model system. At the subcellular level, analysis of actin filament motions in experiments shows that energy dissipated through bending controls the transition between stable and contractile steady states. Using simulations, we show that mechanosensitive binding kinetics of molecular motors controls a fluid-solid phase transition characterized by thermodynamic quantities with opposite symmetries under time-reversal. At the cellular level, we develop new tools for measuring irreversibility in spatiotemporal dynamics to analyze the energetic costs of oscillations and synchronization of a model biochemical oscillator inspired by (dis)assembly driven actomyosin dynamics. Throughout this work, we illustrate that a cell’s distance from equilibrium, quantified by energy dissipation, tunes its mechanical properties and dynamics. This provides a framework to unify disparate biological functions through the lens of non-equilibrium thermodynamics.